Sub.
Code: 212 ‘B’
NEB-GRADE
XII
2076(2019)
Chemistry
Time:
3hrs
Full Marks:75
Pass
Marks: 27 (only for partial students)
Candidates are required to
give their answers in their own words as far as practicable. The figure in the
margin indicates full marks.
Group
‘A’
Attempt
any fifteen questions. 15x2=30
1. How
would you confirm that B in BF3 gets SP2 hybridization?
2. Distinguish
between decinormal and decimolar solution.
3. Find
the PH of 1x10-5 N H2So4.
4. How
many coulombs are required to produce?
i.
27gm of silver from AgNO3?
ii.
50gm of Aluminum from Al2O3?
[At.
Wt. of Ag= 108, At. wt. of Al=27]
5. Define
state function. Write any two examples of it.
6. Calculate
entropy change (ΔS) and free energy change (ΔG) for the conversion of ice into
water at equilibrium condition when enthalpy change (ΔH) is 9KJ/ mol.
7. You
are given a rate law equation, Rat= K [A]2 [B]. By how many times
will the rate increase or decrease for the reaction if
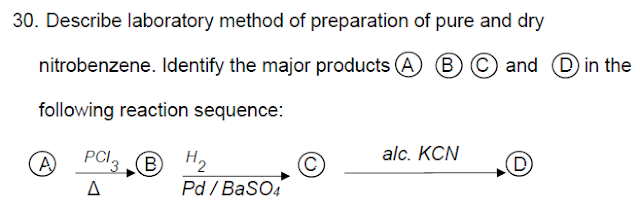
8. Write
down correct reactions for the conversion of ethyne into BHC.
9. Why is
haloarene less reactive than benzene in electrophilic substitution reaction?
10. Starting
from phenol how would you obtain
i.
phenolphthalein
ii.
p-hydroxy azobenzene.
12. An
organic compound C3 H6 O
gives orange precipitate with 2-4 dinitrophenyl hydrazine but does not produce
silver mirror with tollen’s reagent. Identify the compound and write the
reaction involved.
13. Why is
nitro group called an ambident group?
14. Write
an example of each of the following reactions:
i.
Decarboxylation
ii.
Coupling reaction
15. Write
down the functional isomer of methyl methanoate. What product would you expect
when the isomer is heated with P2 O5?
16. How
does DNA differ from RNA in respect of sugar and base units present in it?
17. Define
sugar and non-sugar giving an example of each.
18. Mention
an important function of each of the following:
i.
DDT
ii.
Nitrogen fertilizer
iii.
Broad spectrum antibiotics
iv.
Tranquilizers
19. What
is meant by homo-polymerization? Write an example of such polymer.
20. How is
lithophone prepared? Write its one important application.
21. Why is
silver nitrate solution used for staining fingers of voters during election?
22. What
is meant by
i.
quenching of steel
ii.
annealing of steel
Group ‘B’
Attempt
any five questions.
5x5=25
23. Are
all standard solutions, primary standard solutions or not? Give reason.
I am of a divalent metal was dissolved in 25ml of 2NH2SO4 (f=
1.01). The excess acid required 15.1ml
of 1N-NaOH (f=0.8) for complete neutralization. Find the atomic weight of
the metal.
i.
Draw the standard cell notation.
ii.
Identify the anode and cathode as the current
drawn from it.
iii.
Write the cell reaction taking place at the
electrodes.
iv.
Calculate standard cell potential.
25. Define
bond dissociation energy. The bond dissociation energy of H2(g) and Cl2
(g) are 435 KJ/ mol and 243 KJ
/ mol respectively. The enthalpy of formation of HCl(g) = - 92 KJ/mol. Calculate the bond dissociation energy of HCl(g).
26. How is
blister copper extracted from copper pyrites?
27. Starting
form CH2 Mgl how would you
prepare
i.
Propan-2-ol
ii.
2- methyl propan-2-ol.
28. How is
trichloromethane prepared in the laboratory? Why is chloroform stored in a dark
room air tied bottle containing a little ethyl alcohol?
Group ‘C’
Attempt any two questions.
2x10=20
31. a. How
is ethanal prepared from
i.
ethyne
ii.
But-2-ene
iii.
1, 1-dichloroethane?
Write down suitable method for the conversation
of ethanal into 2-hydroxy propanoic acid.
b.
Write down a structural formula of primary,
secondary and tertiary amine of each from C3 H9 N. How is
Hoffmann’s method applied to separate them from their mixture?
32. Define
the terms
i.
activation energy
ii.
order of reaction
iii.
molecularity of reaction
iv.
effective collision
v.
rate law equation.
Why does powder sugar dissolve
faster than grain sugar?
The following data were
obtained for a hypothetical reaction
Expt.
|
[x]
mol L-1
|
[y]
mol L-1
|
Formation
of z mol L-1 S-1
|
1.
|
0.20
|
0.20
|
3x10-3
|
2.
|
0.40
|
0.20
|
1.2
x10-2
|
3.
|
0.20
|
0.40
|
6x10-3
|
4.
|
0.60
|
0.20
|
9x10-3
|
33. Write short notes on any two.
2x5=10
i.
Chemistry of rusting theory of iron
ii.
Extraction of mercury from cinnabar
iii.
Laboratory preparation of anhydrous formic acid
iv.
Lewis concept of acid and base.
----0----













No comments:
Post a Comment